Professor Guanghui Wang and Haigang Ren's group discovers that glucosylceramide accumulation in microglia triggers STING-dependent neuroinflammation and neurodegeneration
The glucocerebrosidase (GCase) protein, encoded by the GBA gene and localized in lysosomes, is responsible for the degradation of intracellular β-glucosylceramides (GCs). Mutations in the GBA gene are known to be the causative genetic factors for Gaucher disease (GD), a lysosomal storage disorder, and are also considered as major risk factors for Parkinson's disease (PD) and Dementia with Lewy bodies (DLB). Clinical studies have shown that 25% of GD patients with GBA mutations are likely to develop PD, and the risk of cognitive impairment in PD patients with GBA mutations is significantly elevated. Previous research has indicated the involvement of neuroinflammation in the pathology of GD and PD, but the role of GCase in regulating neuroinflammation and its molecular mechanisms remain unclear.
Recently, Professors Guanghui Wang and Haigang Ren from our college, in collaboration with Professors Junhai Han and Ming Fang from Southeast University, published a research paper as a cover article titled Glucosylceramide accumulation in microglia triggers STING-dependent neuroinflammation and neurodegeneration in mice in Science Signaling.

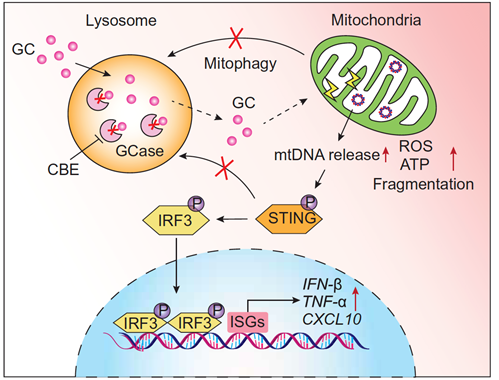
This study revealed that the expression of GCase in microglia in the brain is significantly greater than that in neurons. Inhibiting GCase activity in microglia combined with GC treatment leads to lipid accumulation and activation of STING-dependent inflammation. Mechanistically, GC accumulation in microglia causes mitochondrial dysfunction, resulting in leakage of mitochondrial DNA (mtDNA) into the cytoplasm, triggering STING signaling activation and type I interferon-related inflammatory responses. Additionally, GC accumulation in microglia leads to lysosomal damage, impairing the clearance of damaged mitochondria and hindering their degradation through the lysosomal pathway. Treatment with rapamycin repairs lysosomal damage and mitophagy dysfunction caused by GC accumulation by promoting lysosome biogenesis, thereby ameliorating mitochondrial impairment, cytoplasmic leakage of mtDNA, and STING-dependent inflammatory responses in microglia. Blocking STING activation significantly inhibited microglia-mediated neuroinflammation and protected neurons from neurodegeneration, thereby improving motor behavioral impairments in the mouse model. These findings provide new insights into the pathogenesis of PD and DLB and offer potential drug targets for the clinical treatment of these diseases.
Rui Wang, a doctoral student jointly trained by our college and Southeast University, and Dr. Hongyang Sun, a postdoctoral researcher in our college, are the co-first authors of this study. Professors Guanghui Wang and Haigang Ren from our college and Professors Junhai Han and Ming Fang from Southeast University are cocorresponding authors. This study was supported by the National Natural Science Foundation of China (32261133525, 32271039, 32230039, 32070970, and 32170987) and the Priority Academic Program Development of Jiangsu Higher Education Institutions from Jiangsu Education Department.
Reference:
Rui Wang, Hongyang Sun, Yifan Cao, Zhixiong Zhang, Yajing Chen, Xiying Wang, Lele Liu, Jin Wu, Hao Xu, Dan Wu, Chenchen Mu, Zongbing Hao, Song Qin, Haigang Ren, Junhai Han, Ming Fang, Guanghui Wang. Glucosylceramide accumulation in microglia triggers STING-dependent neuroinflammation and neurodegeneration in mice. Science Signaling. 2024, 17(829): eadk8249.
Full-text link:https://www.science.org/stoken/author-tokens/ST-1774/full
Corresponding authors:

Guanghui Wang is a distinguished professor at the College of Pharmaceutical Sciences, Soochow University. Wang’s laboratory has focused mainly on the molecular basis of neurodegenerative diseases and neurodegeneration in association with neuroinflammation. In recent years, he has published more than 90 academic papers as corresponding author in Sci Adv, Nat Commun, EMBO J, Mol Psychiatry, Autophagy, Aging Cell, Cell Death Differ, Brain, Sci Signal and Cell Rep. To date, these papers have been cited more than 11,500 times.

Haigang Ren is a professor at the College of Pharmaceutical Sciences, Soochow University. Dr. Ren focuses on the molecular mechanisms of Parkinson’s disease and neurodegeneration in association with mitochondrial dysfunction and neuroinflammation. He has published more than 30 academic papers as the corresponding author or first author in Sci Adv, Mol Neurodegener, Sci Signal, Sci China Life Sci, Cancer Lett, J Biol Chem, Acta Pharmacol Sin, Cell Death Dis and Neurosci Bull.