Professors Huabing Chen, Tao Yang and Xiaohui Sun report that TRPV1 blockade selectively amplifies cancer thermo-immunotherapy in Nature Communications
Intrinsic self-defense pathways of tumor cells, such as heat shock proteins (HSPs) upregulation, severely impair therapeutic potencies. Although relevant small-molecule inhibitors have been extensively explored to dismantle self-defenses of tumors for improving therapeutic potencies, such compounds still suffer from severe dose-limiting off-target toxicities owing to their indiscriminate suppression of stressfully overexpressed proteins in tumor and normally expressed proteins in healthy tissues that are necessarily involved in crucial intracellular events such as HSPs-assisted protein folding correction and TGFβ-mediated tissue repair. Hence, exploring a specific and efficient tool to selectively dismantle the self-defenses of tumors is still urgently demanded for safely amplifying cancer therapy.
In recent years, Huabing Chen in the College of Pharmaceutical Science focused on cancer phototherapy and synergistic effects (Adv Mater, 2021, 33, 2004225; Adv Mater, 2021, 33, 2100795; J Control Release, 2022, 350, 761; Adv Mater, 2023, 35, 2210201). Based on the previous research, this team further cooperates with Xiaohui Sun to report that TRPV1 blockade selectively suppresses stressful HSP70 and TGFβ1 via effective modulation of heat shock factor 1 (HSF1) for augmented thermo-immunotherapy against highly malignant tumors.
Firstly, A549-TRPV1 KD cells were constructed to investigate the mechanisms of TRPV1 blockade on calcium influx, HSF1 nuclear translocation, and HSP70 expression (Figure 1A). Hyperthermia resulted in considerable calcium influx, while TRPV1 blockade or knockdown significantly suppressed calcium influx (Figure 1B), thus resulting in the inhibition of HSF1 nuclear translocation and HSP70 upregulation (Figures 1C and 1D). Finally, TRPV1 blockade or knockdown remarkably amplified the antitumor potency of thermotherapy (Figure 1E).
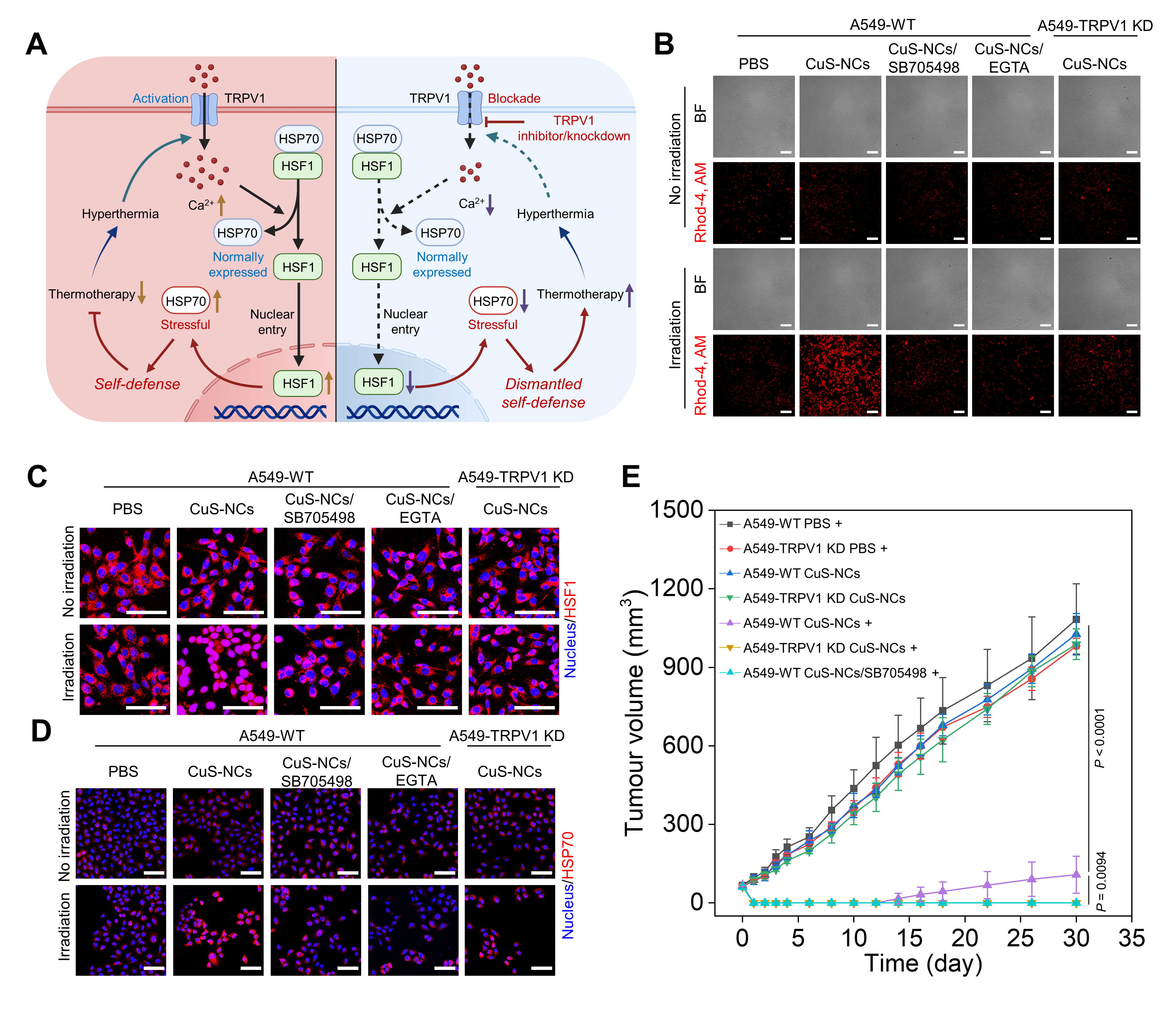
Figure 1 In vitro mechanisms and in vivo antitumor potency of TRPV1 blockade-synergized thermotherapy.
Indocyanine green and TRPV1 antagonist co-encapsulated micelles (IS-Micelles, 42.8 nm) were further synthesized for tumor-targeted delivery (Figure 2A). As a result, IS-Micelles showed remarkable antitumor potencies against large and orthotopic colorectal tumor models(Figure 2B-D). Meanwhile, IS-Micelles displayed negligible influence on the normally expressed HSP70 in healthy tissues, while HSP70 inhibitors obviously downregulated normally expressed HSP70 in healthy tissues (Figure 2E). Moreover, as compared to HSP70 inhibitors, IS-Micelles showed no influence on the heart, liver, and kidney function markers (Figure 2F), revealing good in vivo safety.
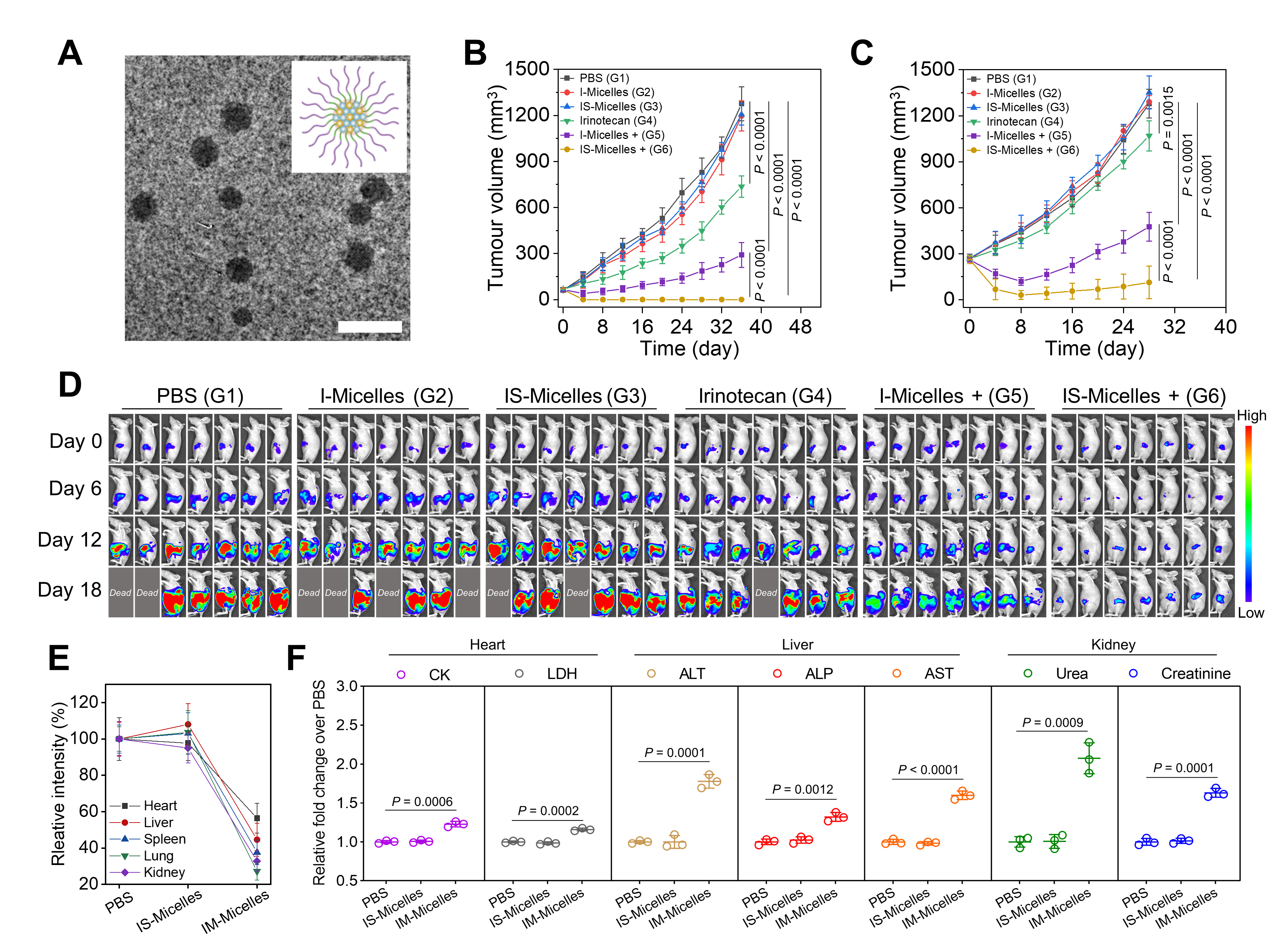
Figure 2 In vivo antitumor potency and safety evaluation of tumor-targeted IS-Micelles.
IS-Micelles also achieved effective modulation of tumor extracellular matrix. TRPV1 blockade-synergized thermotherapy resulted in remarkable downregulation of TGFβ1 expression and cancer-associated fibroblasts and reduced fibrosis (Figure 3A-C), thus leading to improved infiltrations of aPD-L1 for amplifying immunotherapeutic potencies (Figure 3D and 3E).
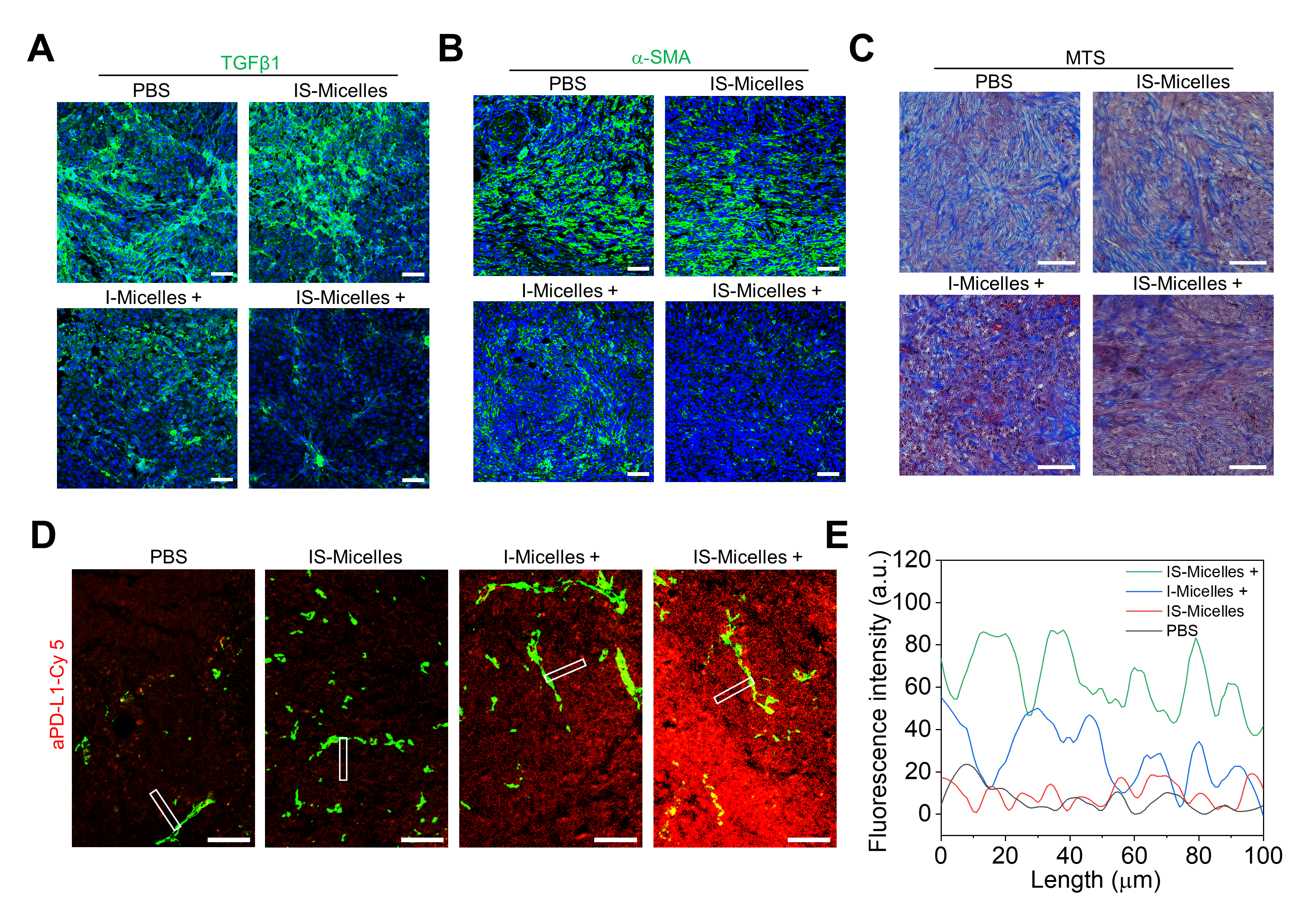
Figure 3 In vivo modulation of tumor extracellular matrix in PDAC models by IS-Micelles.
Clearly, IS-Micelles/aPD-L1 combination remarkably delayed the progression of orthotopic PANC02 tumor models (Figure 4A) and prolonged the survival time (Figure 4B). Meanwhile, TRPV1 blockade-synergized thermo-immunotherapy afforded effective downregulation of MDSCs (Figure 4C), repolarization of M2-like TAMs into M1-like TAMs(Figure 4D), dendritic cells maturation(Figure 4E) and improved infiltration of CTLs and natural killer cells (Figure 4F and 4G), thus remodeling tumor microenvironment to achieve potent thermo-immunotherapy (Figure 4H).
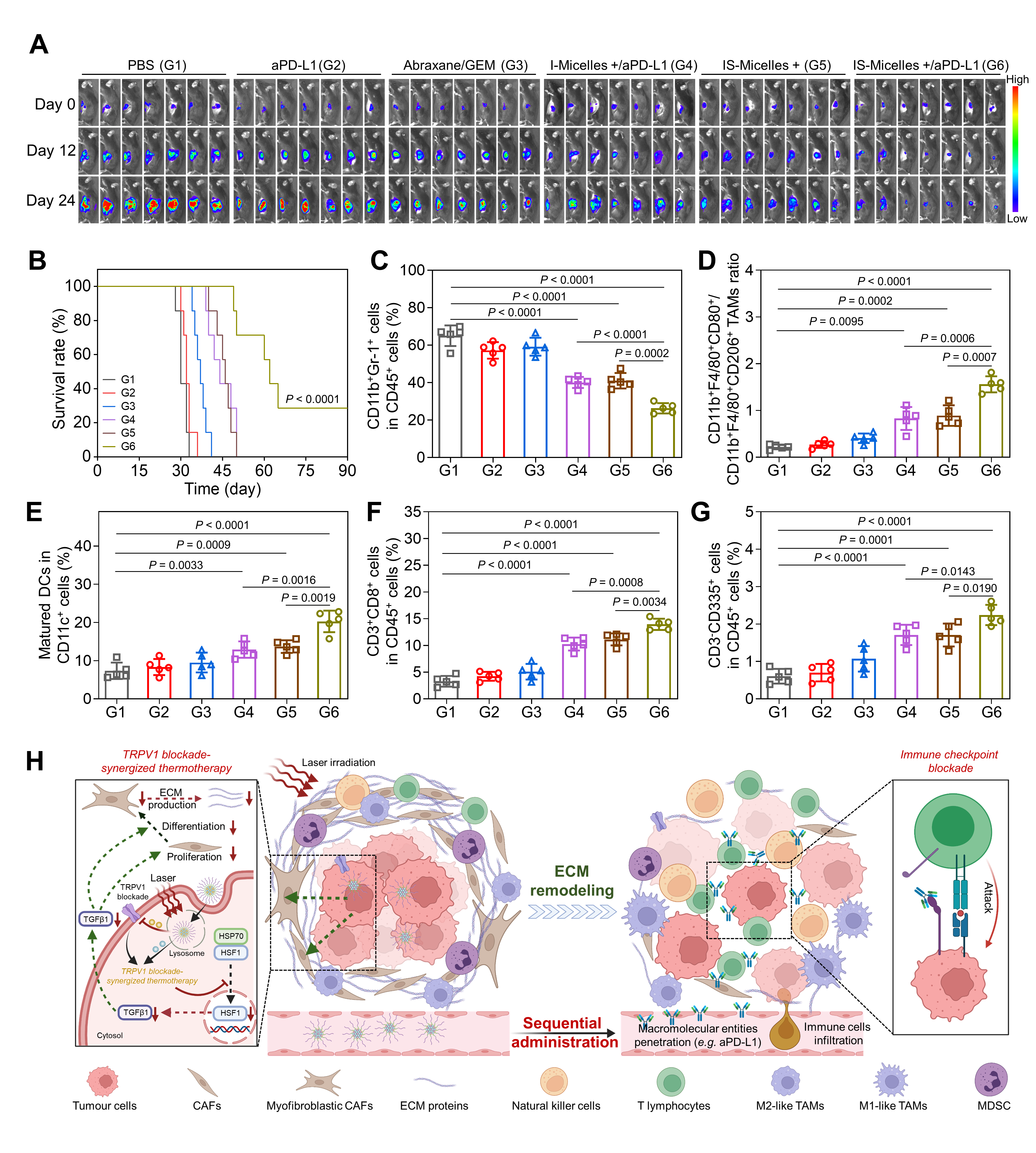
Figure 4 In vivo antitumor potency and immune response of IS-Micelles and immune checkpoint inhibitors in PDAC models.
Such nanoparticles-mediated TRPV1 blockade provides an emerging paradigm to dismantle the self-defenses of tumors for safely amplifying cancer therapy against highly intractable tumors via modulating the highly immunosuppressive and desmoplastic tumor microenvironment.
Reference:Ting Li#, Shuhui Jiang#, Ying Zhang, Jie Luo, Ming Li, Hengte Ke, Yibin Deng, Tao Yang*, Xiaohui Sun*, Huabing Chen*, Nanoparticle-mediated TRPV1 channel blockade amplifies cancer thermo-immunotherapy via heat shock factor 1 modulation. Nat Commun, 2023, 14, 2498. DOI: 10.1038/s41467-023-38128-x