The Huanqiu Li/Sheng Tian Research Group Published a Series of Papers on Intelligent Drug Design Driven by Molecular Dynamics Simulations
Current strategies in targeted drug design are shifting from traditional "broad-spectrum" target intervention toward a deep elucidation of fine-tuned target regulatory mechanisms. This strategy no longer views drug targets merely as simple "on" or "off" switches but aims to reveal and exploit the complex intracellular dynamic regulatory networks of targets, including their conformational diversity, allosteric modulation sites, post-translational modification states, and interaction patterns with other biological macromolecules. Molecular Dynamics (MD) simulations are routinely employed to validate the binding modes of candidate drugs to targets, assess binding stability, and identify critical interacting residues. Due to their ability to perform simulations at microsecond or even millisecond scales in physiological-like environments (e.g., water, lipid membranes, ions), MD plays a pivotal role in artificial intelligence-driven drug design. Recently, the Huanqiu Li/Sheng Tian research group at the Intelligent New Drug Discovery Research Center, School of Pharmacy, Soochow University, leveraging their laboratory's intelligent drug design platform, has achieved a series of advances centered on "MD + Drug Design" in the discovery of small molecule drugs targeting purine receptors and peptide-radionuclide conjugates. These results have been published in Journal of Advanced Research and Journal of Medicinal Chemistry.
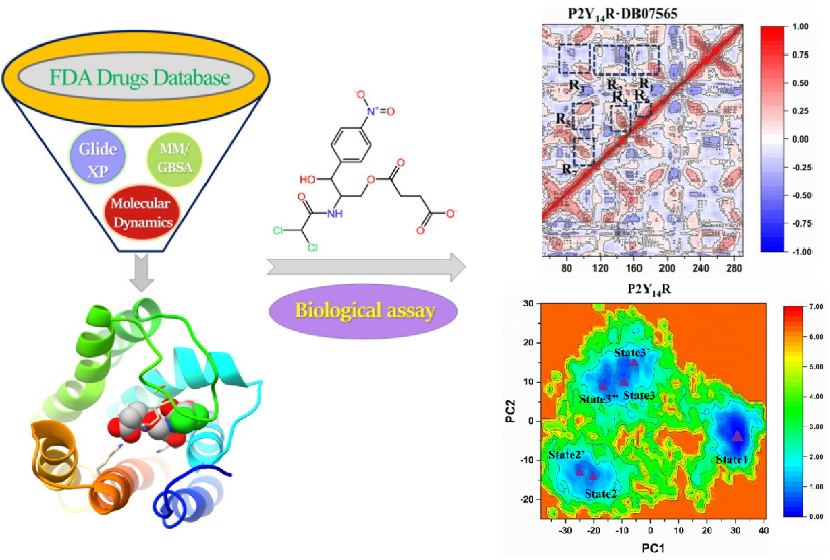
In August 2025, the group, in collaboration with Professor Qinghua Hu's group from China Pharmaceutical University, published a research paper titled "Computational discovery and repurposing of chloramphenicol succinate as a potent P2Y14 receptor antagonist for inflammatory bowel disease therapy" in the top Q1 journal Journal of Advanced Research (IF = 13.0). In this study, MD was not merely a tool for theoretical verification but the core engine driving "drug repurposing" from "serendipitous discovery" to "rational design." It transformed the "probability" based on scoring into "credibility" based on dynamic behavior. Through long-timescale MD simulations of the complex formed by the screened candidate drug and the purine receptor P2Y14R, the research team not only validated its binding stability in a physiological-like membrane environment but also revealed the mechanistic basis by which it induces receptor conformational changes, stabilizes key functional domains (such as TM5/TM6), and forms persistent molecular interactions. This provided a clear direction for subsequent experimental validation. Associate Professor Sheng Tian of the School of Pharmacy, Soochow University, is the first and corresponding author, doctoral candidate Kai Wang is the co-first author, and Professor Huanqiu Li is the corresponding author.
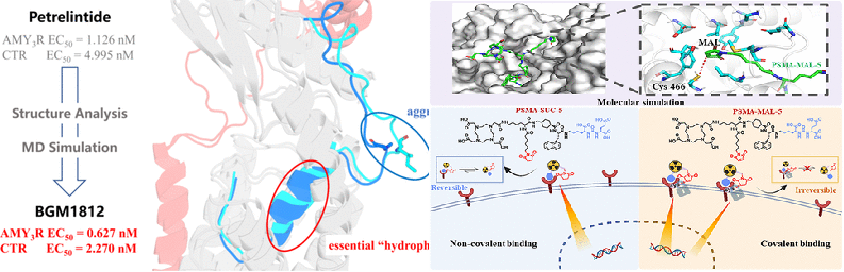
Furthermore, leveraging MD simulation-driven drug design as the core technical support, the Huanqiu Li group recently collaborated with the Guanglin Wang group at the School of Radiation Medicine and Protection, Soochow University, and BrightGene Pharmaceutical Co., Ltd., achieving progress in the design of peptide-radionuclide conjugates and peptide structural optimization. These efforts resulted in two consecutive publications in the top medicinal chemistry journal, Journal of Medicinal Chemistry. In one study, the group utilized MD simulations to identify a covalent binding pocket on the Prostate-Specific Membrane Antigen (PSMA). They designed and synthesized a maleimide-modified targeting peptide, PSMA-MAL-5, capable of forming a "click" covalent bond with PSMA. Upon labeling with 177Lu/225Ac, the conjugate demonstrated significantly enhanced tumor uptake (AUC increased by 4.4-fold compared to 177Lu-PSMA-617) and retention. Even under low-dose 225Ac labeling, it achieved a tumor growth inhibition rate of 80.1 ± 8.9%, providing a novel strategy for precise radionuclide therapy in PSMA-positive prostate cancer (Click Covalent-Targeted Radionuclide Therapy for Prostate Cancer, J. Med. Chem. 2025, 68, 17794-17807).
Addressing the development bottlenecks of Dual Amylin and Calcitonin Receptor Agonists (DACRAs) for obesity treatment, the group used the clinical candidate Petrelintide as a template for optimization. Utilizing structure-based peptide design and MD simulations, they elucidated the "hydrophobic cage" mechanism central to the peptide's binding with the amylin receptor (AMYR) and calcitonin receptor (CTR). Through two rounds of methylation strategies, they designed a novel DACRA agent, BGM1812. This agent demonstrated significantly improved AMY3R/CTR agonist activity in vitro (EC50 reduced by 44.3% and 54.6%, respectively, compared to Petrelintide) and achieved superior weight control and lean mass retention in diet-induced obesity (DIO) rats, offering a new candidate for high-quality obesity treatment (Discovery of BGM1812, a Novel Dual Amylin and Calcitonin Receptor Agonist for Obesity Treatment, J. Med. Chem. 2025, 68, 14907-14918). Doctoral candidate Zhoudong Zhang from the School of Pharmacy, Soochow University, is the co-first author of the above two papers, with Professor Huanqiu Li serving as the corresponding author.
The aforementioned work was supported by the National Natural Science Foundation of China (82373725, 82373887), the Suzhou Science and Technology Plan, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Computational discovery and repurposing of chloramphenicol succinate as a potent P2Y14 receptor antagonist for inflammatory bowel disease therapy. J Adv Res. 2025, 08.22. https://doi.org/10.1016/j.jare.2025.08.035
Click Covalent-Targeted Radionuclide Therapy for Prostate Cancer, J. Med. Chem. 2025, 68, 17794-17807. https://doi.org/10.1021/acs.jmedchem.5c01515
Discovery of BGM1812, a Novel Dual Amylin and Calcitonin Receptor Agonist for Obesity Treatment, J. Med. Chem. 2025, 68, 14907-14918. https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c01120